OTI-2024 (1.5% Eye Drops), Pre-Phase 3 First Curative Therapy for Refractory Glaucoma.

First-in-Class Glaucoma Therapy Powered by MMP Biology
OTI-2024 targets both IOP reduction and neural protection-via IOP Normalization and TM Rejuvenation
Lead Product Dev. Status: IND submitted under 505(b)(2) regulatory pathway, clinical optimal dose (1.5%) defined, Phase 3 primary endpoints (dual) defined: IOP @3 months, and HVF@16 months. Pending CMC and eye drop reformulation.
Development Risk: low. Anticipated on >90% success in pivotal Ph3 trials.
Strong patent protection for the drug use in glaucoma (utility patent) with 20-year market exclusivity and 5-year regulatory exclusivity for both eye drops and SR implant, and MMP platform therapy).
Intellectural Property
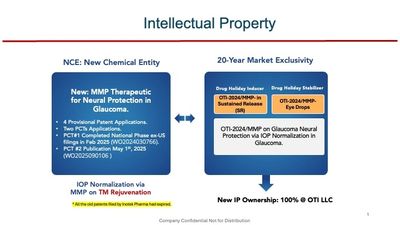
Strong Patent Landscape
OTI holds two synergistic PCTs that together provide comprehensive IP protection for its Trabodenoson/MMP-based glaucoma therapy platform. These patents broadly cover both eye drop and sustained-release (SR) implant products by defining optimal dosing and novel indications such as IOP normalization and retinal ganglion cell (RGC) neuroprotection for glaucoma. MMP therapeutic efficacy has been validated clinically in multiple drugs and laser therapy. Clean ownership, no royalty obligations.
Patent Milestones
1. First Response-Positive Written Opinion
- PCT #1 received a positive written opinion from the PCT office dated Nov 20, 2023, confirming novelty of key claims, including the effective 1.5% dose for the new indication of IOP normalization. PCT# 1 has completed national phase filings to US, EU, Canada, Australia, India, China (6 jurisdictions). Anticipated to be granted as early as 2026.
- PCT #2 received a positive written opinion on June 6, 2024, validating novelty of claims protecting the SR implant (anterior chamber rod) for neuroprotection via IOP normalization, and broader MMP platform therapy for neuroprotection in glaucoma.
2. Provisional Filings:
The original provisional submissions to USPTO include over 180 figures (slides), totaling 103,000 words across 405 pages, was uploaded on USPTO's secure filing system. These patented database layout foundation for patent procecution with success.
Partnering with Us!

1) OTI is actively looking for Pharma/biotech partnership for the lead asset: OTI-2024 (Eye Drops) @Pre Phase 3 (patented), flexible with all forms of collaborations.
2) OTI holds strong patents for the sustained release (SR) implant of OTI-2024 for treating glaucoma neural degeneration, look for out-licensing the SR product patent to technology company.
Contact us: